Our Research
We are working to understand how cells respond to pathogens, and how these signaling pathways can be harnessed for new potential therapies to treat cancer and autoimmune diseases. Importantly, many aspects of the cellular response to infection remain unknown. Our lab uses an approach where we seek to reconstitute signaling outside of the cell using highly purified components in order to understand the mechanisms that control human immunity.
cGAS-STING Signaling
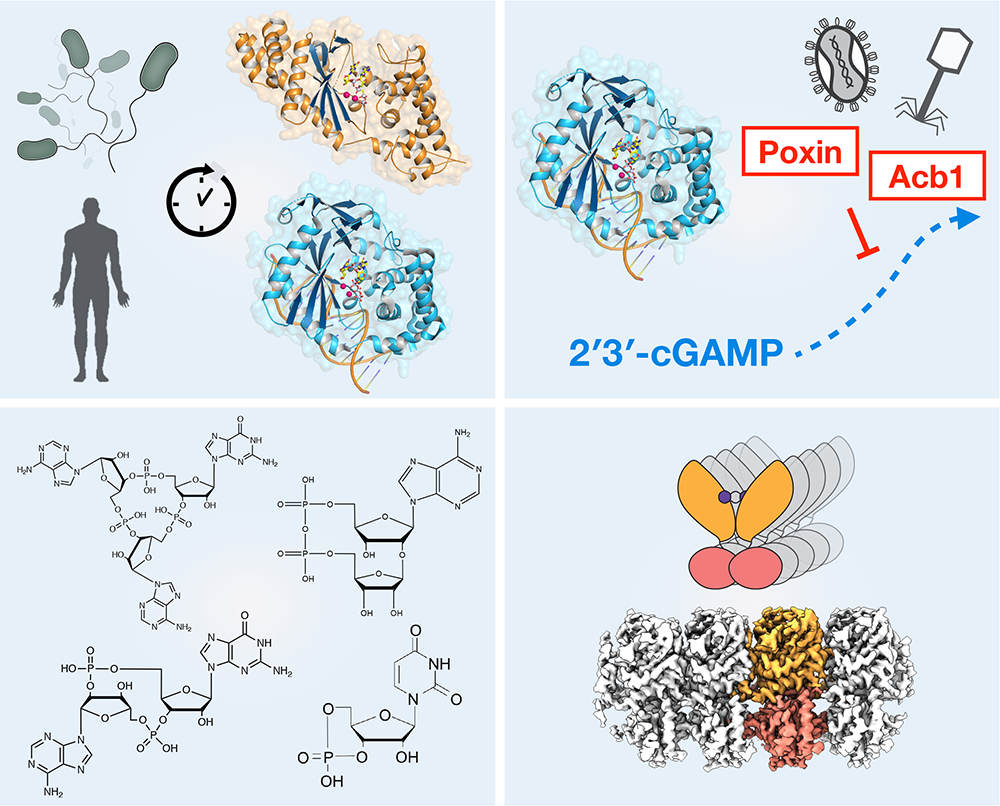
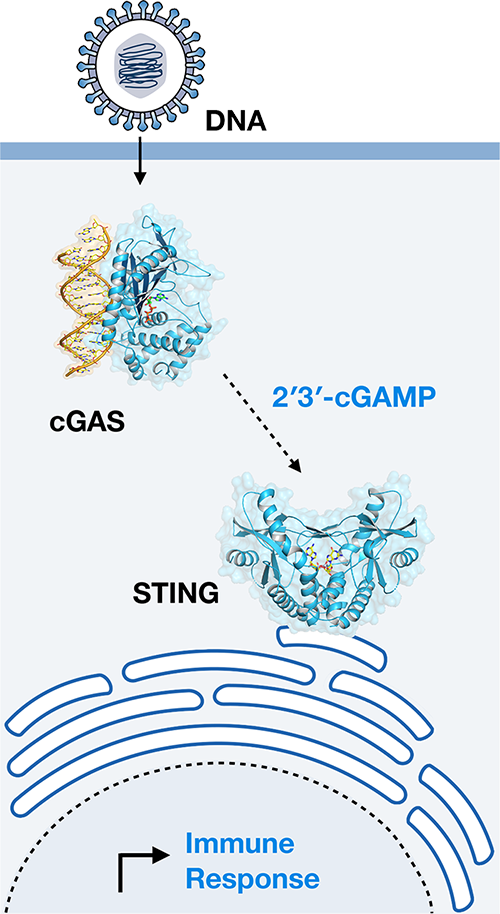
The cGAS-STING signaling pathway is essential for recognition of DNA released into the cell cytosol during pathogen replication, cellular stress, and cancer. Upon recognition of cytosolic DNA, the enzyme cGAS catalyzes formation of cyclic GMP–AMP (2′3′-cGAMP), a specialized nucleotide signal that activates the receptor STING to initiate an immune gene expression program. Due to broad tissue tropism and the ability to potently respond to natural small-molecules, cGAS-STING signaling is an emerging target for cancer immunotherapy. Our research demonstrates that cGAS is part of a large family of animal pattern recognition receptors named cGAS-Like Receptors (cGLRs) and that many innate immune pathways remain to be discovered. Using a structural and biochemical approach, we are working to determine the mechanisms of cGAS-STING and cGLR signaling:
How do cellular and pathogen co-factors regulate cGAS-STING immunity?
How do cGLRs and novel nucleotide signals shape specificity within the human immune system?
Evolution of Antiviral Immunity
Our research reveals the surprising discovery that human innate immunity evolved from ancient pathways in bacteria. Combining structural biology, cell biology, and novel forward biochemical screening approaches, our work explains the mechanism of how human cells recognize infection and demonstrates that the core components that control these signaling pathways are descended from proteins in bacteria responsible for anti-phage defense. The discovery of the ancient origins of antiviral immunity provides a new framework to explain organization of the human immune system and to define novel components that inhibit pathogen replication in animal cells. We are continuing to build on the discovery of the ancient origins of human immunity with the major goals to:
Discover new pathways that control the host response to infection.
Determine the structural basis of how bacterial and metazoan receptors sense viral replication.
Define the rules that explain how bacteriophages and human viruses defeat host immunity.